commentary
amsa
Fatty Acid Composition of Meat Animals as Flavor Precursors
By Thu T. N. Dinh1*, K. Virellia To2, and M. Wes Schilling2
1. Department of Animal and Dairy Science, Mississippi State University, Mississippi State, MS 39762, USA
2 Department of Food Science, Nutrition, and Health Promotion, Mississippi State University, Mississippi State, MS 39762, USA
*thu.dinh@mssstate.edu
Fatty Acid Composition of Meat Animals
Animal fat includes subcutaneous, intermuscular, and intramuscular (marbling) adipose tissues (Rhee et al., 2000), containing mostly triglycerides (neutral lipids) and a small amount of phospholipids (polar lipids). In lean meat, phospholipids account for up to one-third of marbling. Fatty acids in meats are unbranched and have an even number of carbons from 4 to 24, although 12 to 24 is the most common (Voet et al., 2006). However, some branched or odd-numbered fatty acids can be found in ruminant fat (Lobb and Chow, 2000). Lamb has up to 3% volatile branched-chain fatty acids (Bravo-Lamas et al., 2018), contributing to characteristic lamb and mutton odors. Longer branched-chain fatty acids in beef do not contribute to flavor.
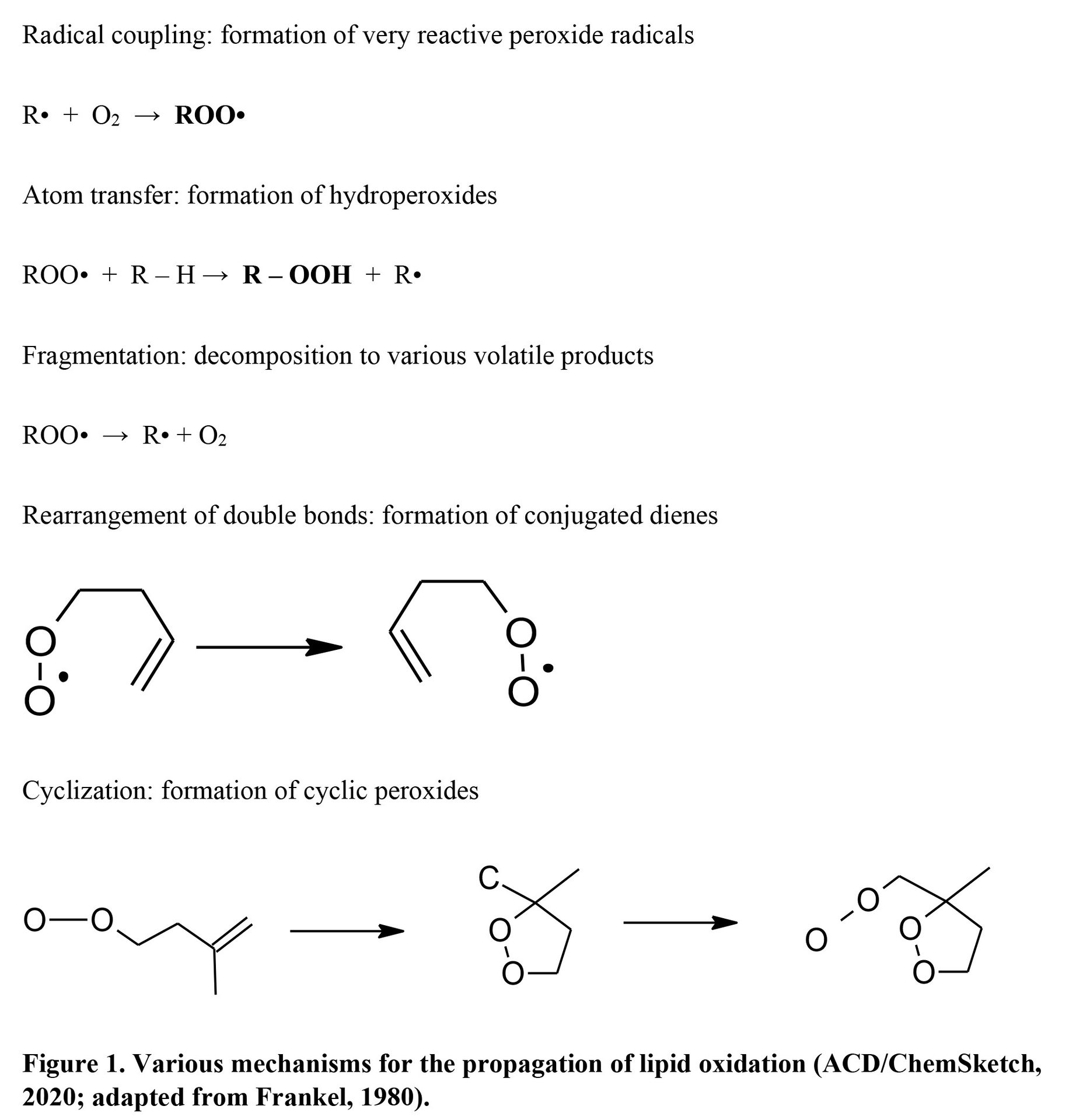
Fatty Acid Oxidation and Cooked Meat Aroma
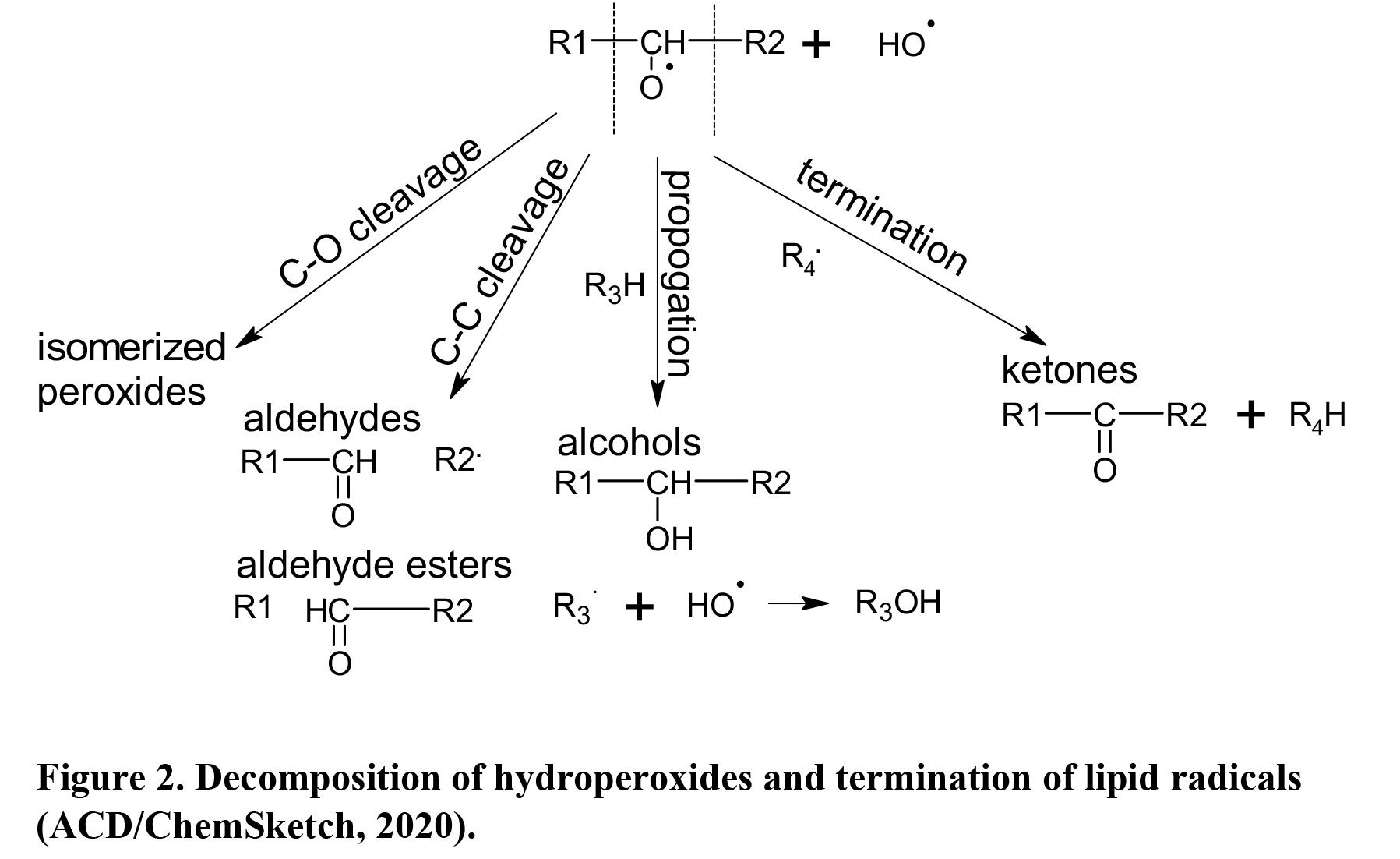
The abstraction of the hydrogen at an allylic carbon adjacent to the C=C double bond in fatty acids produces lipid radicals, which couple with oxygen and produce hydroperoxides. Lipid peroxides undergo structural changes (Figure 5) and are decomposed into various aldehydes, esters, alcohols, ketones, carboxylic acids, and hydrocarbons (Figure 8). yielding undesirable aromatic profiles during storage (Domínguez et al., 2019) but a desirable ones during cooking under thermal oxidation (Nawar, 1984). Song et al. (2011) reported that hexanal, 1-octen-3-ol, (E,E)-2,4-decadienal, and (E,E)-2,4-heptadienal are characteristic of beef flavor in addition to butanoic, 2-methylbutanoic, 3-methylbutanoic, heptanoic, 4-ethyloctanoic, and nonanoic acids (Um et al., 1992). However, other n-aldehydes and unsaturated aldehydes such as (E)-2-nonenal, 4-heptenal, nonanal, octanal, and (E)-2-decenal are undesirable. Studies in beef and pork have consistently identified hexanal and 2,3‐octanedione as warmed-over flavor markers. Pork-characteristic volatiles are slightly different, including ethyl acetate, 3‐(methylthio) propanal, hexanal, 2‐butanone, dimethyl disulfide, and dimethyl trisulfide. Lamb-characteristic volatiles result from alkylpyrazines, such as 2,5-dimethylpyrazine, and alkylpyridines, such as 2-pentylpyridine (Buttery et al., 1977); both are products of lipid-Maillard interactions. Lamb volatiles also contain more saturated aldehydes than goat meat and other meats (Madruga et al., 2013). Unsaturated fatty acids, more predominant in polar lipid fraction, are oxidized more during cooking (Legako et al., 2015). Elmore et al. (1999) increased n-3 PUFA in beef muscle and produced up to 4-fold more undesirable lipid oxidation products, particularly n-alkanals, 2-alkenals, 1-alkanols, and alkylfurans. Mottram and Edwards (1983) also reported that polar lipids, not neutral lipids, are more important for cooked beef aroma.
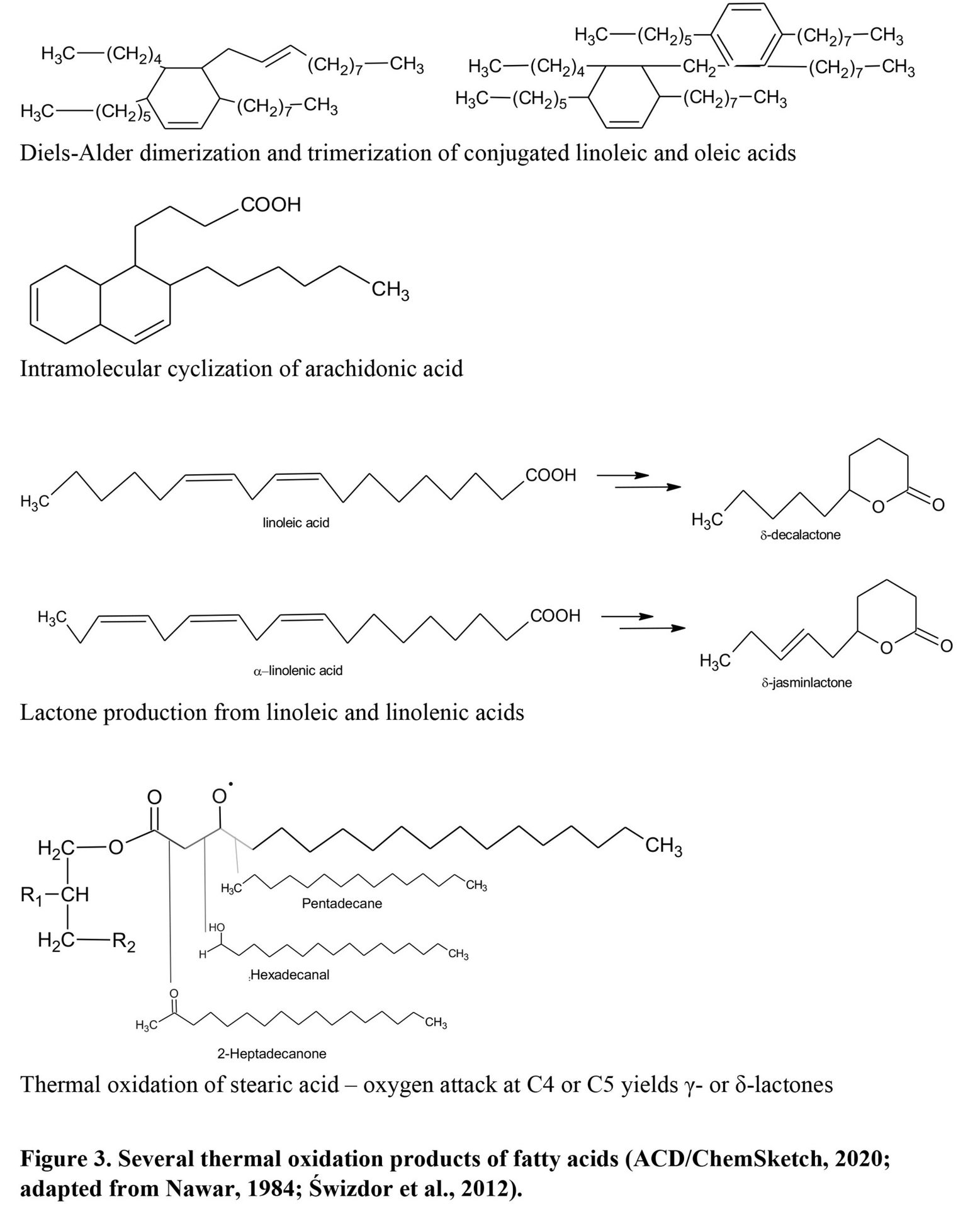
Thermal oxidation of lipids occurs through similar mechanisms and produces the same classes of compounds as autoxidation (Choe and Min, 2007). Although it can occur at as low as 60°C, the desirable composition of lipid-derived volatiles is produced at a temperature from 100°C up to 300°C (Wasserman, 1972) because these conditions create more lactones, alcohols, ketones, and short-chain fatty acids. High temperature and available oxygen during cooking drive volatile composition to a more desirable direction with rapid and further oxidation to organic acids and esters (Choe and Min, 2007; Song et al., 2011), greater polymerization of unsaturated fatty acids and lipid peroxides to produce fewer volatile products, and degradation of SFA to long-chain alkanes, aldehydes, and lactones (Nawar, 1984), especially at 150°C or above - such as on the surface of grilled steaks (Figure 9). However, lipid thermal oxidation products such as cyclic compounds and fatty acid dimers are also decomposed to offensive aromas during storage.
As fatty acids on the surface of roasted meat are oxidized extensively at 190°C, milder Maillard reactions occur at an internal temperature of 60°C to 80°C (Wasserman, 1972), in which lipid-derived aldehydes are active participants, yielding some of the most characteristic volatiles of cooked meat aroma. Many short-chain aldehydes and ketones are also removed by the steam during cooking, decomposed under high temperature, and react with Maillard reaction products, to produce more desirable volatile compounds with much lower thresholds.
Fatty Acid Oxidation and Maillard Reactions
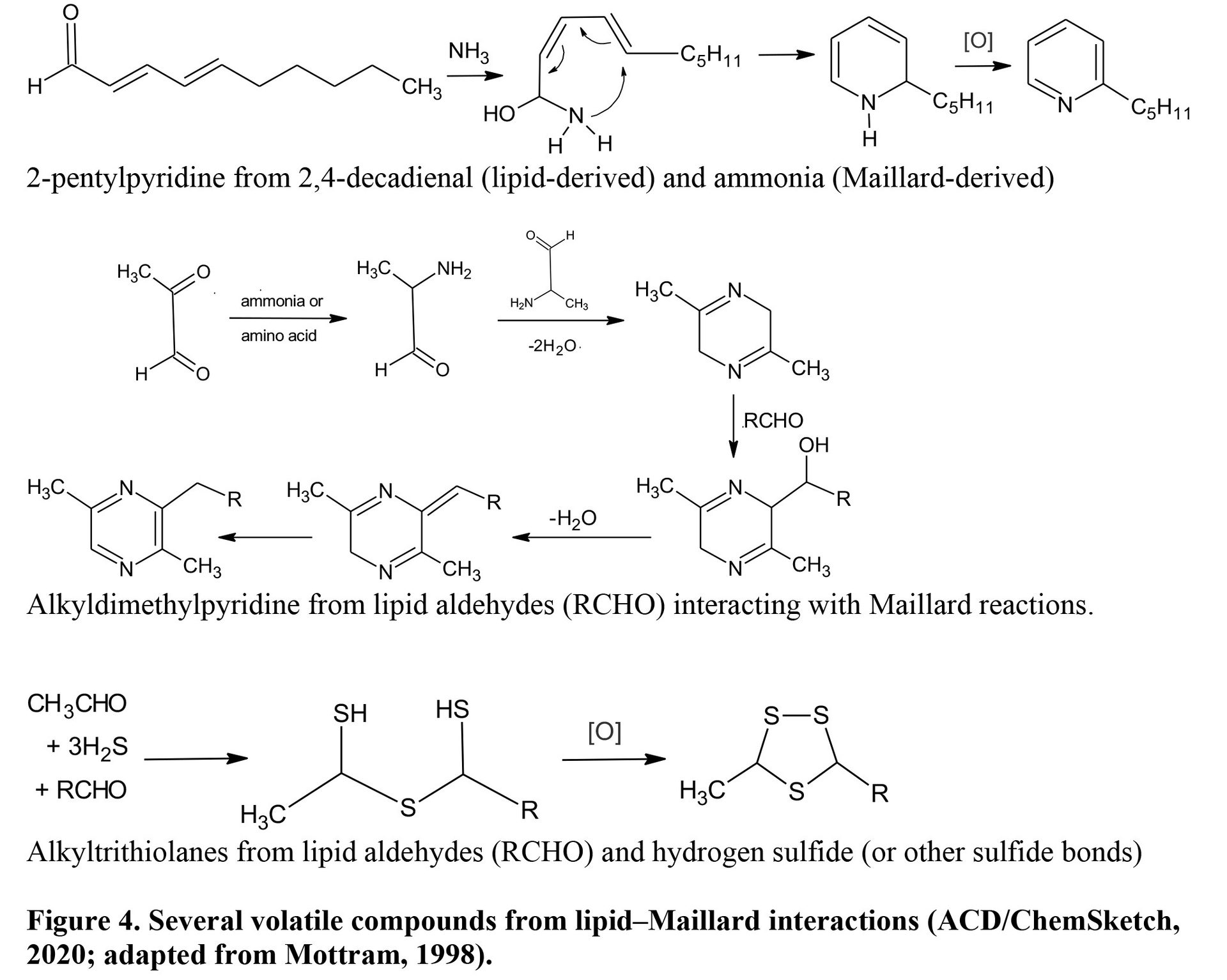
Lipid-Maillard interactions were recognized early in meat flavor chemistry research as pathways to produce some of the key flavor compounds in cooked meat. Fat extract from pork tissues with chloroform and methanol developed species-specific meat flavor after heating but such flavor notes diminished after the extract was washed with water (Wasserman and Spinelli, 1972), leaving only a “piggy” note identified as 5,α-androst-16-en-3-one, the boar odor dissolved in pork fat. Pippen and Mecchi (1969) similarly demonstrated that lipids from chicken adipose tissues had no chicken aroma after removing polar compounds by water washing. Sanderson et al. (1966) also reported that heating beef fat with beef lean yielded much more flavor carbonyls than only beef fat. Myers et al. (2009) suggested that beef and pork lean was more important for species-specific flavor, although fat level affected flavor intensity. Some lamb-specific flavors also come from lipid-Maillard interaction products such as alkylthiazoles, alkylpyrazines, and alkylpyridines (Mottram, 1998). Lipid oxidation products such as aldehydes compete for amino compounds with carbonyls derived from reducing sugars (Zamora and Hidalgo, 2011). Alkyldimethylpyrazines and alkyltrithiolanes are formed as lipid-derived aldehydes enter Maillard reactions with either pyrazines or hydrogen sulfide, respectively (Figure 10). Lipid aldehydes and ammonia, a byproduct of Maillard reactions, form 2-pentylpyridine (Figure 10), which recently was reported to produce various characteristic and desirable flavor compounds in cooked meat (Kosowska et al., 2017). Lipid-Maillard interactions occur at a greater intensity at higher internal temperatures above 66°C (Myer et al., 2009) and in fattier meat because of the availability of lipid oxidation precursors (Gardner and Legako, 2018). Fatty acid oxidation products are not simply aromatic compounds; they are also precursors for complex interactions with Maillard products to form a more characteristic and desirable cooked meat aroma. Such interactions do not occur in autoxidation during the storage of meats.
Conclusions
Volatile compounds from autoxidation of unsaturated fatty acids causes off-odors, whereas the lipid-derived volatile profile is more desirable under thermal oxidation and in the reactions with Maillard reaction products. Recent research has suggested that the development of lipid flavor compounds is influenced by the lean portion of meat. Therefore, the interactions between lipid-derived compounds, water-soluble compounds, and Maillard compounds are likely more important than originally thought and warrant further research.
Literature Cited
Bravo-Lamas, L., L. J. Barron, J. K. Kramer, I. Etaio, and N. Aldai. 2016. Characterization of the fatty acid composition of lamb commercially available in northern Spain: Emphasis on the trans-18: 1 and CLA content and profile. Meat Sci. 117:108–116. https://doi.org/10.1016/j.meatsci.2016.02.043.
Buttery, R. G., L. C. Ling, R. Teranishi, and T. R. Mon. 1977. Roasted lamb fat: Basic volatile components. J. Agr. Food Chem. 25:1227–1229. https://doi.org/10.1021/jf60214a038.
Choe, E., and D. B. Min. 2007. Chemistry of deep‐fat frying oils. J. Food Sci. 72:R77–R86. https://doi.org/10.1111/j.1750-3841.2007.00352.x.
Domínguez, R., M. Pateiro, M. Gagaoua, F. J. Barba, W. Zhang, and J. M. Lorenzo. 2019. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants. 8:429. https://doi.org/10.3390/antiox8100429.
Elmore, J. S., D. S. Mottram, M. Enser, and J. D. Wood. 1999. Effect of the polyunsaturated fatty acid composition of beef muscle on the profile of aroma volatiles. J. Agr. Food Chem. 47:1619–1625. https://doi.org/10.1021/jf980718m.
Frankel, E. N. 1980. Lipid oxidation. Prog. Lipid Res. 19:1–22. https://doi.org/10.1016/0163-7827(80)90006-5.
Gardner, K., and J. F. Legako. 2018. Volatile flavor compounds vary by beef product type and degree of doneness. J. Anim. Sci. 96:4238–4250. https://doi.org/10.1093/jas/sky287.
Kosowska, M., M. A. Majcher, and T. Fortuna. 2017. Volatile compounds in meat and meat products. Food Sci. Tech.-Brazil. 37:1–7. https://doi.org/10.1590/1678-457X.08416.
Legako, J. F., T. T. N. Dinh, M. F. Miller, and J. C. Brooks. 2015. Effects of USDA beef quality grade and cooking on fatty acid composition of neutral and polar lipid fractions. Meat Sci. 100:246–255. https://doi.org/10.1016/j.meatsci.2014.10.013.
Lobb, K., and C. K. Chow. 2000. Fatty acid classification and nomenclature. In: C. K. Chow, editor, Fatty acids in foods and their health implications. CRC Press, Boca Raton, FL. pp. 1–16.
Madruga, M., I. Dantas, A. Queiroz, L. Brasil, and Y. Ishihara. 2013. Volatiles and water-and fat-soluble precursors of Saanen goat and cross Suffolk lamb flavour. Molecules. 18:2150–2165. https://doi.org/10.3390/molecules18022150.
Mottram, D. S. 1998. Flavour formation in meat and meat products: A review. Food Chem. 62:415–424. https://doi.org/10.1016/S0308-8146(98)00076-4.
Mottram, D. S., and R. A. Edwards. 1983. The role of triglycerides and phospholipids in the aroma of cooked beef. J. Sci. Food Agr. 34:517–522.
Myers, A. J., S. M. Scramlin, A. C. Dilger, C. M. Souza, F. K. McKeith, and J. Killefer. 2009. Contribution of lean, fat, muscle color and degree of doneness to pork and beef species flavor. Meat Sci. 82:59–63. https://doi.org/10.1002/jsfa.2740340513.
Nawar, W. W. 1984. Chemical changes in lipids produced by thermal processing. J. Chem. Educ. 61:299. https://doi.org/10.1021/ed061p299.
Pippen, E. L., and E. P. Mecchi. 1969. Hydrogen sulfide, a direct and potentially indirect contributor to cooked chicken aroma. J. Food Sci. 34:443–446. https://doi.org/10.1111/j.1365-2621.1969.tb12800.x.
Rhee, K. S., D. F. Waldron, Y. A. Ziprin, and K. C. Rhee. 2000. Fatty acid composition of goat diets vs intramuscular fat. Meat Sci. 54:313–318. https://doi.org/10.1016/s0309-1740(99)00094-7.
Sanderson, A., A. M. Pearson, and B. S. Schweigert. 1966. Effect of cooking procedure on flavor components of beef. Carbonyl compounds. J. Agr. Food Chem. 14:245–247. https://doi.org/10.1021/jf60145a013.
Song, S., X. Zhang, K. Hayat, P. Liu, C. Jia, S. Xia, Z. Xiao, H. Tian, and Y. Niu. 2011. Formation of the beef flavour precursors and their correlation with chemical parameters during the controlled thermal oxidation of tallow. Food Chem. 124:203–209. https://doi.org/10.1016/j.foodchem.2010.06.010.
Świzdor, A., A. Panek, N. Milecka-Tronina, and T. Kołek. 2012. Biotransformations utilizing β-oxidation cycle reactions in the synthesis of natural compounds and medicines. Int. J. Mol. Sci. 13:16514–16543. https://doi.org/10.3390/ijms131216514.
Voet, D., J. G. Voet, and C. W. Pratt. 2006. Fundamentals of biochemistry: Life at the molecular level. Wiley, Hoboken, NJ. p. 1264.
Wasserman, A. E. 1972. Thermally produced flavor components in the aroma of meat and poultry. J. Agr. Food Chem. 20:737–741.
Wasserman, A. E., & Spinelli, A. M. (1972). Effect of some water-soluble components on aroma of heated adipose tissue. Journal of Agricultural and Food Chemistry, 20(2), 171-174.
Zamora, R., and F. J. Hidalgo. 2011. The Maillard reaction and lipid oxidation. Lipid Technology. 23:59–62. https://doi.org/10.1002/lite.201100094.
